There is one factor that can have a MAJOR impact on blood sugar stability that many in the diabetic space are completely unaware of.
Do you know what that is?
Your gut.
For approximately 20+ years, the gut has gained more recognition for its direct and indirect connections with specific health conditions, illnesses, and diseases.
Let’s take a closer look at how your gut health may add to some type 1 diabetic (T1D) frustrations!
Understanding Your Gut Microbiome
The gut microbiome refers to the community of microorganisms, including bacteria, viruses, fungi, and other gut microbes that reside within your gastrointestinal (GI) tract.
Gut researchers commonly refer to these microbes as “gut bugs” for simplicity.
This collection of gut bugs plays various essential roles in proper bodily functions, such as:
- Digestion and nutrient absorption (main function)
- Keeping pathogens away alongside your immune system (main function)
- Metabolism regulation (secondary function)
By the way, all of these are common impairments among T1Ds!
A healthy gut microbiome is characterized by having more good gut bugs than bad ones, along with a wide variety of beneficial microbes that contribute to better overall health for you and your gut (1, 2).

However, various factors can disrupt this balance and lead to a condition known as gut dysbiosis.
I will now do a short review of some of these variables.
However, if you’re unfamiliar with the following gut health basics, I strongly encourage you to review this series of gut posts I’ve written. I go into more detail about each category and more!
In addition, I describe their association with type 1 diabetes development.
Diet
What you’re eating can significantly impact your gut microbiome.
Generally, a fiber-rich diet of fruits, vegetables, and fermentable foods can support a diverse and healthy gut (3).
However, this may be a little complicated for type 1 diabetics who follow a low-carb diet because many of the existing fiber-rich foods are also typically high in carbohydrates.
Conversely, a diet high in processed foods, sugars, and low-to-minimal nutrients can also promote the growth of harmful gut bugs (4).
Great.
So, does this mean you can’t eat anything?
Nope.
I review some of these low-carb shortcomings and potential solutions for you and your diabetic physician review.
Chronic Use of Antibiotics, PPIs, and NSAIDs
The chronic use of antibiotics may impair the gut microbiome considerably (5).
While antibiotics are crucial for treating bacterial infections, they can also disrupt the delicate balance of microbes in your gut.

Though antibiiotics are intended to get rid of the bad bacteria present, it unfortuantely also eliminates many of the good bacteria bugs, as well!
While the intention is to attack the underlying bacterial trigger, antibiotics may become problematic if:
- They’re used with long-term consistency (time will vary)
- Bacteria isn’t the source of the problem
- The problem is bacterial, but the antibiotics have now compromised the gut microbiome while eliminating the pathogen
The chronic use of certain medications is also strongly associated with disrupting the gut microbiome, such as
(6, 7):
- proton pump inhibitors (PPIs) that are used to reduce stomach acid and
- non-steroidal anti-inflammatory drugs (NSAIDs)
Stress
Chronic stress can influence the gut microbiome through the gut-brain axis, a bidirectional communication system between the gut and the brain.

- alter gut motility (the pushing of food throughout your gut during digestion)
- increase leaky gut risk
- disrupt the balance of gut bugs in your microbiome
Due to this gut dysfunction, insulin resistance may then worsen if it’s already present.
Let’s review how this chain reaction takes place.
1. Stress-Induced Dysbiosis
Chronic stress may promote gut dysbiosis, favoring the growth of bad gut bugs associated with inflammation and insulin resistance.
Stress-induced dysbiosis may contribute to the impairments listed above, such as inflammation and changes in short-chain fatty acid production (I’ll review SCFAs shortly!).
2. Hormonal Impact
Stress may lead to the release of stress hormones, such as cortisol and epinephrine, which can alter gut function and microbe balance (10).
These hormones can affect insulin sensitivity directly and indirectly by signaling the production and release of more glucose for energy during these stressful events, even if you don’t need it.
3. Appetite and Dietary Choices
Stress can also influence dietary choices, leading individuals to prefer comfort foods that are often highly processed with little (if any) nutritional value.
These dietary choices can contribute to gut dysbiosis and worsen insulin resistance (11, 12).
Physical Inactivity
Lack of exercise can create an environment in the gut that favors the growth of bad gut bugs while reducing the good ones.
This imbalance may contribute to inflammation and metabolic problems, including insulin resistance and weight gain (13).
Regular physical activity promotes a healthier gut microbiome by promoting microbial diversity and a more balanced gut-bug community.
This typically has a positive effect on overall health (14).
Diseases
Medical research has established a strong likelihood that gut dysbiosis may be present well before autoimmunity shows signs of its presence (15).
However, once autoimmunity is established, this increases further gut microbe impairment and disease risk (16, 17).
Insulin Resistance, Your Gut, and High Blood Glucose Levels
Insulin resistance is when the body’s muscle, fat, liver, and other cells become less responsive to insulin (18).
As a result, the cells have difficulty taking up glucose from the bloodstream, leaving it in the blood, and leading to elevated blood sugars or hyperglycemia.
This consistent state of hyperglycemia increases the risk of many different diabetic complications or other ill conditions (19).

But as I’ve stated in many other posts, to address what’s going on, you must first understand why it’s not working in the first place.
Now, let’s dig into some mechanisms through which gut dysbiosis may contribute to insulin resistance, higher blood sugars, and unreliable diabetic management.
Inflammation and Endotoxemia
Inflammatory Mechanism
We know that dysbiosis can lead to an overgrowth of bad gut bugs while reducing the levels of beneficial, anti-inflammatory gut bugs.
This shift in the microbial balance leads to, at minimum, low-grade chronic inflammation in the gut, leading to a condition often called gut permeability or leaky gut (20).
In a leaky gut, the intestinal barrier becomes less strict in what it allows to pass through into the blood.
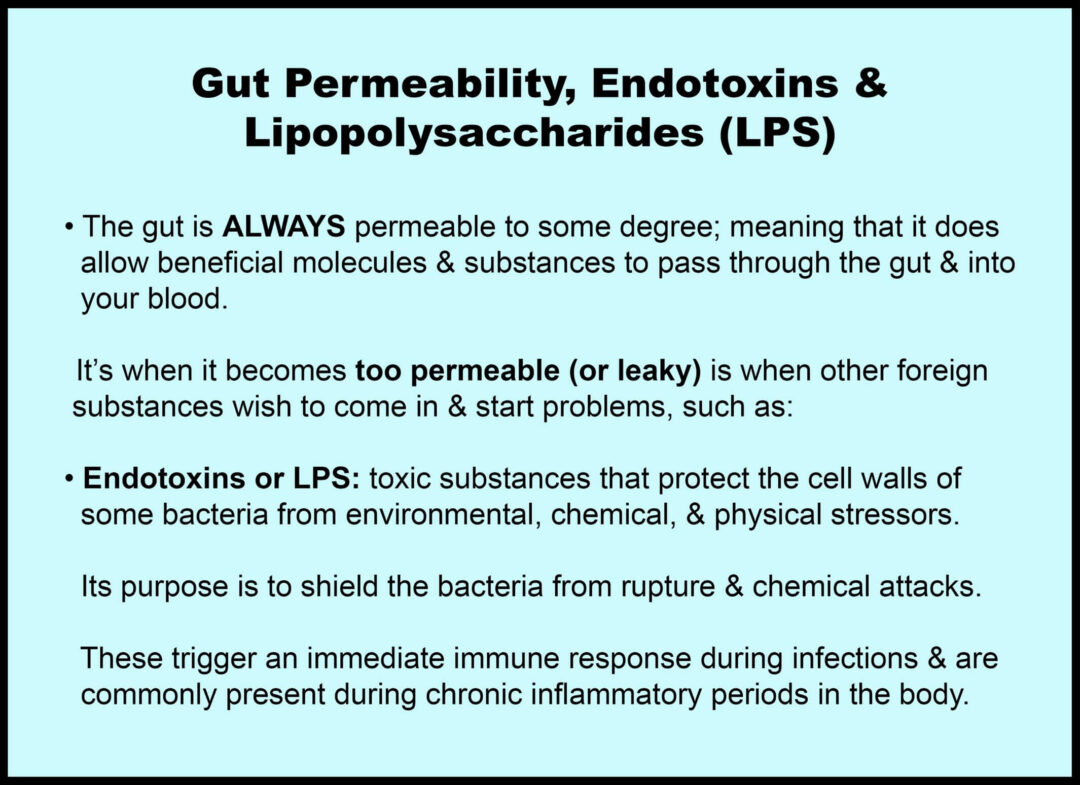
A leaky gut allows substances like endotoxins and LPS (these two words are commonly used interchangeably) to enter the bloodstream.
Once in the bloodstream, LPS can trigger immune responses and systemic inflammation and reprogram the body to store more fat.
This chronic, low-level inflammation can contribute to insulin resistance in 2 ways (21, 22):
- By disrupting insulin signaling in various tissues, including muscle, liver, and fat cells
- Increasing body fat composition, which also further encourages insulin resistance
Chronic inflammation may interfere with insulin’s ability to regulate glucose uptake consistently by the body cells, leading to troubled blood sugars.
Reduced Short-Chain Fatty Acids (SCFAs)
SCFA Mechanism
Beneficial gut microbes ferment dietary fiber to produce short-chain fatty acids, such as butyrate, propionate, and acetate.
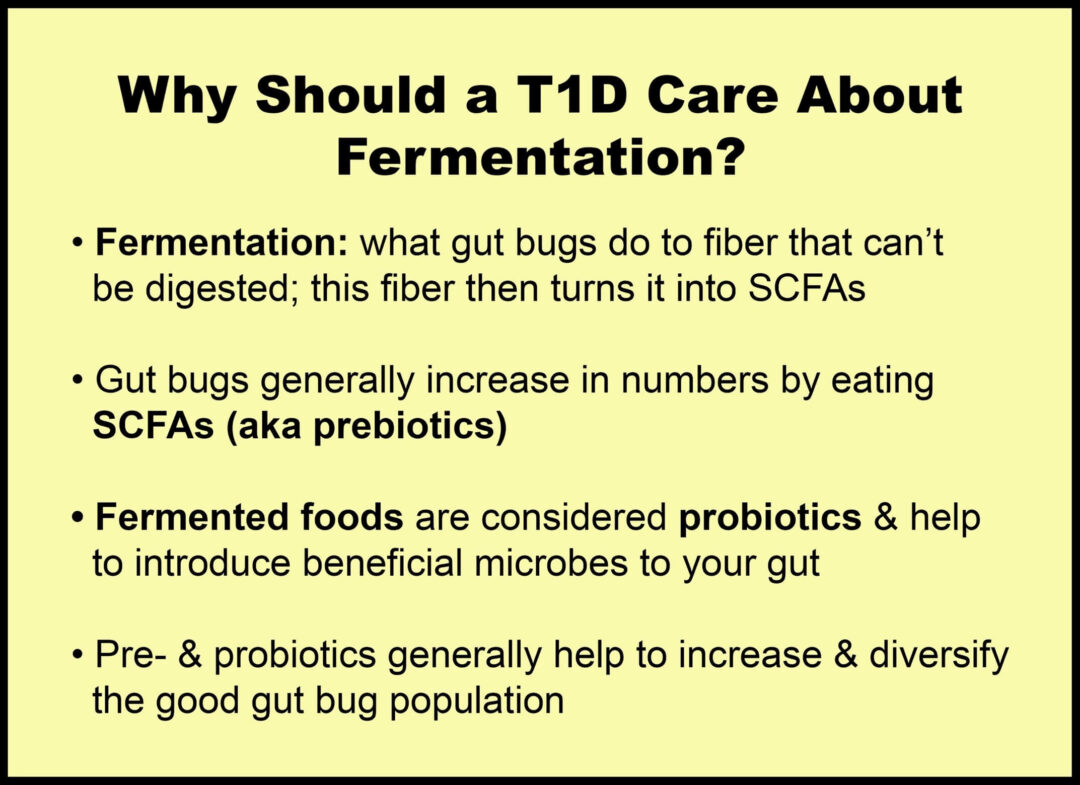
As a result, SCFAs have been shown to play a crucial role in promoting insulin sensitivity by:
- increasing glucose uptake
- reducing glucose production in the liver
- improving hormonal regulation
However, it’s already been noted that gut dysbiosis generally lowers SCFA content in the body.
These low levels of SCFAs may encourage insulin resistance as follows:
- Reduced Glucose Uptake
Low SCFAs may impair insulin signaling due to inflammation.
Gut inflammation is common with low levels of SCFAs, especially if gut dysbiosis is present, and will likely lead to systemic inflammation if not treated properly.
Inflammation generally alters insulin signaling and reduces insulin’s ability to stimulate glucose uptake among the cells that need it (23).
- Increase Glucose Production in the Liver
The liver is the primary organ for glucose metabolism (take care of it!).
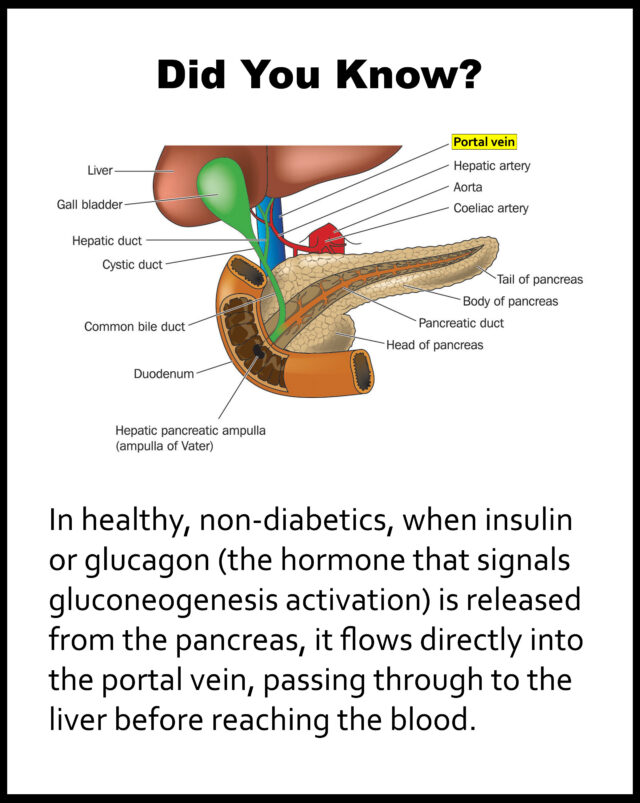
One of the ways the gut microbiome and the liver communicate is via the portal vein, which transports SCFAs from the gut to the liver (24, 25).
SCFAs can act as a signaling molecule influencing liver function (26).
When SCFA levels are low, this may disrupt this communication and affect glucose metabolism.
One way this may occur is through impaired gluconeogenesis, the process by which the liver produces glucose from non-carbohydrate sources.
Gluconeogenesis is usually suppressed when we eat because we don’t need to add more glucose to the blood during meals, right?
When SCFAs are not up to par, this may reduce the inhibition of gluconeogenesis, leading to increased glucose production in the liver (27).
This may now reduce insulin sensitivity and increase insulin resistance.
- Impaired Regulation of Hormones
SCFAs can influence the release of glucose-stabilizing gut hormones.
Did you know that insulin is a hormone?
These gut hormones assist with insulin secretion and glucose regulation (28).
When SCFAs are low, this may reduce glucose regulating efficiency.
Hormone dysregulation may also negatively affect glucose uptake in the cells and alter liver function related to glucose metabolism and regulation.
The dysfunction doesn’t stop here, though.
Another aspect of liver function may continue another vicious circle via bile acids.
Let’s go over this now.
Bile Dysfunction
You’re now aware of one way the liver may alter glucose regulation, but there is another way!
Bile is a greenish-yellow fluid of waste products, cholesterol, and bile salts.
It is produced and released by the liver through many bile ducts, and stored in the gall bladder.
Bile has two primary functions:
- To carry away waste
- To break down fats into fatty acids
Bile also assists with metabolic regulation.
Consider bile acids as the “fuel” to help bile function accordingly.
The gut microbiome plays a role in bile acid metabolism (29).
If the gut is compromised, this may also affect bile function, leading to insulin resistance.
Impaired Bile Mechanisms
Gut dysbiosis can alter bile acid composition and signaling (30).
Bile acid dysfunction affects several metabolic pathways and signaling mechanisms crucial for maintaining insulin sensitivity.
Here are a few notable ways bile dysfunction may lead to insulin resistance:
- Altered Bile Acid Composition and Metabolism
Remember, one of the main functions of the gut is to absorb nutrients.
When gut dysbiosis is present, impairing bile acid function is one of the specific ways it may impair absorption where fats and fat-soluble vitamins aren’t being absorbed appropriately, thereby causing an inflammatory response.
This is now a three-fold response where:
1. Lipid profiles may increase as more free fatty acids enter the bloodstream (31).
Also, note that excess glucose can quickly turn into fat. So, please don’t think this is a “Stay away from fat!” promo because it’s not, as long as you’re not hypersensitive to it.
As we already know, increased fat elevates insulin resistance risk.
2. Chronic inflammation is the one thing that’s involved with any illness, disease, and sickness.
Unfortunately, poor lipid profiles and inflammation don’t help reduce insulin resistance.
3. Lastly, bile acid dysfunction may also contribute to elevated glucose production in the liver (32).
This embodies another vicious circle!
- Impaired Fat Digestion and Absorption
Bile is essential for the digestion and absorption of dietary fats and fat-soluble vitamins. In cases of bile dysfunction, the impaired digestion and absorption of fats can lead to changes in lipid profiles and increased free fatty acids in the bloodstream.
These changes can cause insulin resistance by interfering with insulin signaling pathways.

Dysregulation of Appetite and Energy Metabolism
Appetite & Energy Mechanism
The gut microbiome influences the secretion of hormones that regulate appetite and energy metabolism (33).
Dysbiosis can alter the balance of these hormones, which may lead to changes in appetite, food intake, and energy expenditure (34).
Altered gut hormone levels may affect insulin sensitivity and glucose metabolism, as changes in appetite and energy expenditure can influence:
- overeating
- hyperinsulinemia (too much use of insulin)
- increased weight gain
- elevated body fat production
These are critical factors in developing insulin resistance (35).
How Your Gut & Insulin Resistance May Impact Rollercoaster Blood Sugars (aka Glycemic Variability)
I go over more information regarding the literal ups-and-downs of unstable blood sugar episodes in my Roller Coaster Blood Sugars: What Causes Them & Why Should All Type 1 Diabetics Care? post if you wish to learn more about this topic.
Medical research is limited in directly linking gut health to rollercoaster blood sugars.
However, finding direct associations between insulin resistance and glycemic variability is not (36)!
One observational study even found more reliable glycemic variability risk among T1D subjects, but only when they had insulin resistance (37).
A minimum of 33% of all type 1 diabetics are suggested to have insulin resistance (38).
This estimation increases to at least 50% if you’ve had T1D for at least ten years, though this is likely an understatement (39)!
At this point, we know that gut dysbiosis strongly influences impaired insulin function, resulting in insulin resistance.
The gut microbiota plays a pivotal role in digesting carbohydrates, producing short-chain fatty acids, and regulating gut hormones, all of which influence how our body manages blood sugar.
With dysbiosis, the gut’s carbohydrate metabolism can become altered, resulting in:
- Reduced SCFA Production
- Altered gut hormone secretion
- Increased leaky gut risk
In addition to hyperglycemia, this combined effect of all these disruptions may also lead to rollercoaster blood sugars as insulin will likely not work with the precision it needs.

The main takeaway for you all to know is that troubled blood sugars and insulin resistance are typically a domino effect of lifestyle behaviors that do not favor healthy and reliable diabetic management.
Most of the time, it is a choice we can control. However, it may be a challenging choice to follow through with.
It just depends on how sick and tired you are of being sick and tired.
Your health and longevity are worth it!
Summary
- Gut health has an immense role in blood sugar regulation, yet many diabetics are completely unaware of its beneficial impact on overall BG stability.
- Gut dysfunction can influence poor blood sugar through:
- Gut dysbiosis
- Cortisol impairment
- Hormonal disruption
- Increased leaky gut risk
- Reduced short-chain fatty acids, which are extremely beneficial for gut and blood glucose stability
- Organ dysfunction
- Hunger dysregulation
- All of these can collectively add to insulin resistance’s instability
References
2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8954387/
3. https://www.hsph.harvard.edu/nutritionsource/microbiome/
4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6835660/
5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9959899/
6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10159235/
7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4754147/
8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2714186/
9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7213601/
10. https://www.frontiersin.org/articles/10.3389/fendo.2023.1130689/full
11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6835660/
12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3705322/
13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8877435/
14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5357536/
15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9632986/
16. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6854958/
17. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)00457-9/fulltext
18. https://www.ncbi.nlm.nih.gov/books/NBK507839/
19. https://www.cdc.gov/diabetes/managing/problems.html
20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8100306/
21. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2246086/
22. https://diabetesjournals.org/diabetes/article/56/7/1761/12590/Metabolic-Endotoxemia-Initiates-Obesity-and
23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2246086/
24. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6319369/
25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9105144/
26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8007165/
27. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8835596/
28. https://pubmed.ncbi.nlm.nih.gov/32887215/
29. https://www.cell.com/trends/microbiology/fulltext/S0966-842X(22)00286-4
30. https://gut.bmj.com/content/62/4/531
31. https://pubmed.ncbi.nlm.nih.gov/2184846/
32. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5789421/
33. https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-021-01093-y
34. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8877435/
35. https://diabetesjournals.org/care/article/31/Supplement_2/S262/24841/Insulin-Resistance-and-HyperinsulinemiaIs
36. https://pubmed.ncbi.nlm.nih.gov/31707004/
37. https://pubmed.ncbi.nlm.nih.gov/35657731/
38. https://link.springer.com/article/10.1007/s13300-019-00729-5
39. https://onlinelibrary.wiley.com/doi/abs/10.1002/dmrr.3640